This is a VERY IMPORTANT video for anyone doing Western Blotting (i.e. everyone!).
Archive for the ‘Western blot tutorial seriesâ Category
Can We Trust Western Blots?
:: Posted by American Biotechnologist on 07-08-2014Video: How to Perform Perfect Quantitative Western Blotting
:: Posted by American Biotechnologist on 04-01-2014Keeping it constant: A lesson in protein transfer
:: Posted by American Biotechnologist on 05-22-2012 Power supplies that are used for electrophoresis hold one parameter constant (either voltage, current, or power). The PowerPac⢠HC and PowerPac Universal power supplies also have an automatic crossover capability that allows the power supply to switch over to a variable parameter if a set output limit is reached. This helps prevent damage to the transfer cell.
Power supplies that are used for electrophoresis hold one parameter constant (either voltage, current, or power). The PowerPac⢠HC and PowerPac Universal power supplies also have an automatic crossover capability that allows the power supply to switch over to a variable parameter if a set output limit is reached. This helps prevent damage to the transfer cell.
During transfer, if the resistance in the system decreases as a result of Joule heating, the consequences are different and depend on which parameter is held constant.
Transfers Under Constant Voltage
If the voltage is held constant throughout a transfer, the current in most transfer systems increases as the resistance drops due to heating (the exception is most semi-dry systems, where current actually drops as a result of buffer depletion). Therefore, the overall power increases during transfer, and more heating occurs. Despite the increased risk of heating, a constant voltage ensures that field strength remains constant, providing the most efficient transfer possible for tank blotting methods. Use of the cooling elements available with the various tank blotting systems should prevent problems with heating.
Transfers Under Constant Current
If the current is held constant during a run, a decrease in resistance results in a decrease in voltage and power over time. Though heating is minimized, proteins are transferred more slowly due to decreased field strength.
Transfers Under Constant Power
If the power is held constant during a transfer, changes in resistance result in increases in current, but to a lesser degree than when voltage is held constant. Constant power is an alternative to constant current for regulating heat production during transfer.
The above information was adapted from Bio-Radâs protein blotting guide. For more great information, be sure to download the Protein Blotting Guide from Bio-Rad Laboratories.
Top 8 reasons for high background noise on your western blot
:: Posted by American Biotechnologist on 05-02-2012 The following are the top 8 causes for overall high background on your western blot and potential solutions:
The following are the top 8 causes for overall high background on your western blot and potential solutions:
- Blocking was incomplete
- Increase the concentration of blocker
- Increase the duration of the blocking step
- Use a different blocking agent
- Blocker was impure
- Use a pure protein such as BSA or casein as a blocker
- Wash protocols were insufficient
- Increase the number, duration, or stringency of the washes
- Include progressively stronger detergents in the washes; for example, SDS is stronger than Nonidet P-40 (NP-40), which is stronger than Tween 20
- Include Tween 20 in the antibody dilution buffers to reduce nonspecific binding
- The blot was left in the enzyme substrate too long (colorimetric detection)
- Remove the blot from the substrate solution when the signal-to-noise level is acceptable, and immerse in diH2O
- Contamination occurred during electrophoresis or transfer
- Discard and prepare fresh gels and transfer solutions
- Replace or thoroughly clean contaminated foam pads if a tank blotter was used
- Excessive amounts of protein were loadedon the gel or too much SDS was used inthe transfer buffer. Proteins can pass through the membrane without binding and recirculate through a tank blotting system.
- Reduce the amount of protein on the gel or SDS in the transfer buffer
- Add a second sheet of membrane to bind excess protein
- The primary or secondary antibody was too concentrated
- Increase antibody dilutions
- Perform a dot-blot experiment to optimize working antibody concentration
- Incubation trays were contaminated
- Clean the trays or use disposable trays
For more great information, download the protein blotting guide from Bio-Rad Laboratories.
A primer on fluorescence detection
:: Posted by American Biotechnologist on 01-31-2012Yesterday we told you about how to get more data from your western blots by utilizing multiplex fluorescent detection. Today, we will provide you with a primer on fluorescent detection taken from the Bio-Rad Laboratories Protein Blotting Guide.
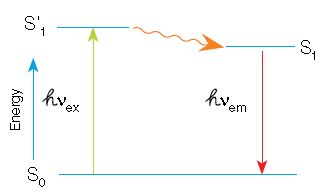
In fluorescence, a high-energy photon (âVex) excites a fluorophore, causing it to leave the ground state (S0) and enter a higher energy state (Sâ1). Some of this energy dissipates, allowing the fluorophore to enter a relaxed excited state (S1). A photon of light is emitted (âVem), returning the fluorophore to the ground state. The emitted photon is of a lower energy
(longer wavelength) due to the dissipation of energy while in the excited state.
When using fluorescence detection, consider the following optical characteristics of the fluorophores to optimize the signal:
- Quantum yield â efficiency of photon emission after absorption of a photon. Processes that return the fluorophore to the ground state but do not result in the emission of a fluorescence photon lower the quantum yield.Fluorop hores with higher quantum yields are generally brighter
- Extinction coefficient â measure of how well a fluorophore absorbs light at a specific wavelength. Since absorbance depends on path length and concentration (Beerâs Law), the extinction coefficient is usually expressed in cmâ1 Mâ1. As with quantum yield, fluorophores with higher extinction coefficients are usually brighter
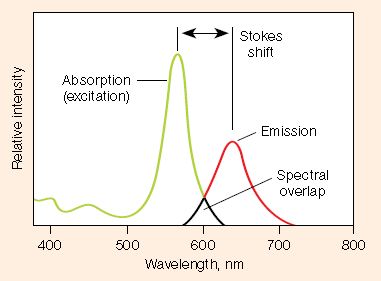
- Stokes shift â difference in the maximum excitation and emission wavelengths of a fluorophore. Since some energy is dissipated while the fluorophore is in the excited state, emitted photons are of lower energy (longer wavelength) than the light used for excitation. Larger Stokes shifts minimize overlap between the excitation and emission wavelengths, increasing the detected signal
- Excitation and emission spectra â excitation spectra are plots of the fluorescence intensity of a fluorophore over the range of excitation wavelengths; emission spectra show the emission wavelengths of the fluorescing molecule. Choose fluorophores that can be excited by the light source in the imager and that have emission spectra that can be captured by the instrument. When performing multiplex western blots, choose fluorophores with minimally overlapping spectra to avoid channel crosstalk
For more information be sure to download the Protein Blotting Guide from Bio-Rad Laboratories.