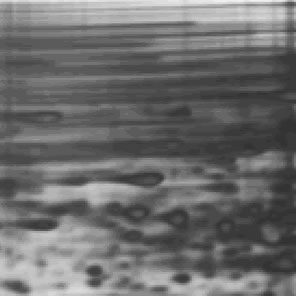
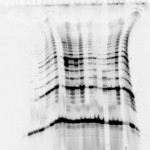
Last week’s ugly gel was one of the ugliest 2D gels that we’ve seen in a while. There was capacious amounts of horizontal streaking across the top of the gel which made it nearly impossible (OK….completely impossible) to discern discrete spots necessary for protein identification.
Some likely causes for such ugliness include:
1. Protein Overloading
2. Proteins not properly and stably solubilized
3. DNA contamination
4. Incomplete focusing
In order to rectify this problem, it is important to ensure that all proteins are completely solubilized by using a strong chaotropic extraction reagent. The concentrations of urea, thiourea, detergents, carrier ampholytes, and reducing agents (DTT or TBP) are also critical. Every sample type typically requires a new sample preparation method. A good starting point for sample preparation includes the following standard buffer solutions:
8—9 M urea, 4% (w/v) CHAPS, 2 mM TBP or 1% (w/v) DTT, 40 mM Tris, 0.2% (w/v) Bio-Lyte (ampholyte), pH 3—10
7 M urea, 2 M thiourea, 4% (w/v) CHAPS, 2 mM TBP or 1% (w/v) DTT, 40 mM Tris, 0.2% (w/v) Bio-Lyte (ampholyte), pH 3—10
ReadyPrep sequential extraction kit reagent 3: 5 M urea, 2 M thiourea, 2% CHAPS, 2% SB 3—10, 40 mM Tris, 0.2% (w/v) Bio-Lyte (ampholyte), pH 3—10
ReadyPrep 2-D rehydration/sample buffer 1: 7 M urea, 2 M thiourea, 1% ASB-14, 40 mM Tris
Allow sufficient time for full denaturation and solubilization. Allow the sample to sit at room temperature in the solubilization solution for 1 hr before applying to IEF.
Remove insoluble protein complexes by centrifugation (>10,000 x g) prior to IEF.
Luckily, Bio-Rad can help you with this as well. Bio-Rad’s ReadyPrep protein extraction kit and sequential extraction kits make it easy to extract the types of proteins you need quickly and cleanly without contamination from DNA or other artifacts. Yes…2D electrophoresis can be complicated, but with the proper techniques you will end up with clean looking gels that provide you a tremendous amount of data for your proteomics studies.